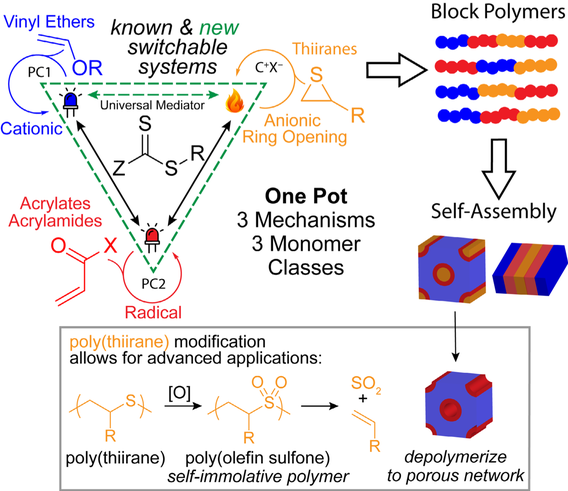
Triply-Switchable Polymerization for the Synthesis of Highly-Ordered Block Polymers
Advanced polymeric architectures require techniques that can tolerate the incorporation of diverse monomer classes; however, current methods to achieve materials from multiple polymerization mechanisms generally require tedious synthetic manipulations on a multi-functional initiator or the chain end to achieve block polymers, often in multiple pots. This proposal seeks to realize higher-level control over block composition and sequence through radical, cationic, and anionic polymerization in one pot, regulated through orthogonal external stimuli. This triply-switchable system will allow for the synthesis of polymers with unique block sequences for the creation of novel architectures, which we anticipate will exhibit distinctive properties. We will extensively study these materials in the context of block polymer self-assembly and by leveraging the unique opportunities presented by the poly(thiirane) block, including oxidation to chiral (sulfoxide) or self-immolative (sulfone) materials and the post-polymerization modification to cationic or zwitterionic states.
New Routes to Carbon Dioxide-based Polyurethanes
Polyurethanes (PUs) are industrially important polymers, but the most common syntheses involve toxic isocyanate precursors. Carbon dioxide would be an ideal C1 source for non-isocyanate polyurethanes (NIPUs) due to its low cost, high abundance, and nontoxic nature. Previous syntheses of NIPUs with CO2 either have low CO2 incorporation or introduce non-carbamate heteroatoms into the backbone, drastically changing the properties of the material. In this proposal, we seek an alternative route to NIPUs from alkyne amines using gold(I) catalysts. The modular nature of the alkyne amine monomers and the resultant pendant alkene moieties will enable the synthesis of a broad range of linear and crosslinked materials. Since chain-growth mechanisms to PUs are rare, we will further explore gold(I) complexes containing Z-type ligands (i.e. σ-acceptor ligands) to maintain association of the growing polymer chain end to the catalyst center throughout the polymerization. Such a mechanism would allow for the synthesis of PUs with unprecedented control, such as the synthesis of gradient copolymers or well-defined block polymers.
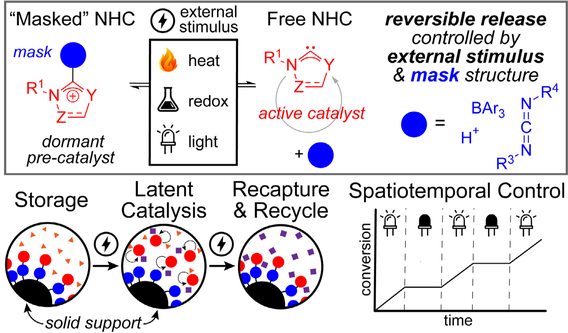
Masked N-heterocyclic Carbenes for Switchable Organocatalysis
N-heterocyclic carbenes (NHCs) are versatile and powerful organocatalysts for both small molecule chemistry and polymerizations. The innate sensitivity of NHCs has prompted the use of reversible NHC adducts (i.e. “masked” NHCs) as easily-handled precursors, but common masks (e.g. CO2) have limited tunability for controlled release. Likewise, current on-off “switchable” NHC catalysts rely on modification of the NHC itself, which inherently changes the reactivity of the catalyst and limits the scope of NHC-catalyzed reactions amenable to these methods. In this project, we seek to design fully-reversible NHC adducts with tunable masks that will enable externally-stimulated release, independent of NHC reactivity. Through this method, a larger selection of on-off switchable NHCs will be explored. These fully reversible adducts will not only allow for latent catalysis, but also catalyst recycling on a solid support as well as spatiotemporal control over polymerizations.